Unit 33: The
Ideal Gas Law
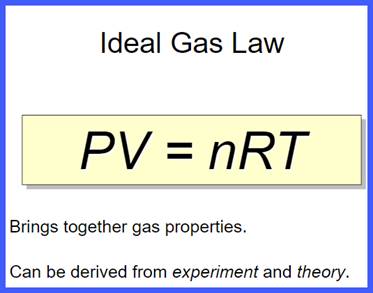
Unit Overview:
In
the last unit, you applied the kinetic molecular theory to the behavior of
gases, exploring their mathematical descriptions. These mathematical descriptions are known as empirical
gas laws.
In
this unit, you will synthesize these empirical gas laws into the ideal gas law.
Let’s Review:
What variables are used to explain gases?
Four variables are used to describe gases: amount,
volume, temperature, and pressure.
|
Variable |
Symbol |
Unit |
|
Amount |
n |
Mole (mol) |
|
Volume |
V |
Liters (L) |
|
Temperature |
T |
Kelvin (K) |
|
Pressure |
P |
Atmosphere (atm) |
What empirical
gas laws describe them?
When any two of these variables are held constant, it
is possible to determine the relationship between the remaining two
variables. These relationships between
two variables are referred to as the empirical gas laws:
|
Empirical
Gas Law |
Held
Constant |
Variables |
Relationship |
Equation |
|
|
Boyle’s law |
Amount of gas Temperature |
Pressure Volume |
inverse |
P . V = constant |
|
|
Charles’ law |
Amount of gas Pressure |
Temperature Volume |
direct |
V T |
= constant |
|
Gay-Lussac’s law |
Amount of gas Volume |
Temperature Pressure |
direct |
P T |
= constant |
|
Avogadro’s law |
Temperature Pressure |
Amount of gas Volume |
direct |
V n |
= constant |
What is the
Ideal Gas Law?
The ideal gas law mathematically combines the four
empirical gas laws together into one equation.
This equation relates all four of the variables that describe a gas into
one equation. This equation defines the
constant and allows us to relate the 4 variables to each other for any sample
of gas.
|
Ideal Gas
Law: |
PV
= nRT |
||
|
P |
Pressure |
Atmosphere (atm) |
|
|
V |
Volume |
Liters (L) |
|
|
n |
Amount of gas |
Mole (mol) |
|
|
R |
Universal Gas Constant |
0.0821 |
L . atm K . mol |
|
T |
Temperature |
Kelvin (K) |
|
Please note:
The units for the universal gas law are very specific. So, when using the ideal gas law to solve
problems, it is important that the units for all of the variables match.
Let’s look at an example together:
|
A
50.0 L tank contains hydrogen (H2) gas at a temperature of 298 K
and a pressure of 1.01 atm. How many moles of hydrogen gas is in the tank? |
||
|
Step 1: Identify all the variables; make sure all
the units are as specified in the table above. P = 1.01 atm V = 50.0 L n = ? mol R = 0.0821 L.atm/K.mol T = 298 K |
||
|
Step 2: Using the ideal gas law equation, solve
for the variable which does not have a value. PV
= nRT In this case, it is “n.” To solve for “n,” divide both sides of the
equation by RT. |
||
|
PV
RT |
= |
nRT RT |
|
This allows the RT/RT to cancel on the right side, leaving “n” by
itself. |
||
|
PV
RT |
= |
n |
|
Step 3: Plug in your known values and solve for
the missing value. |
||
|
(1.01
atm)(50.0 L) (0.0821
L.atm/K.mol) (298 K) |
= |
n = 2.06 mol H2 |
Watch the following video to hear a verbal description of how to solve problems using the ideal gas law:
Practice 1: Complete the online problem-solving quiz of the ideal
gas law.
What is an
ideal gas?
Notice that we refer to this combination of the
empirical gas laws as the ideal gas law. Why do we use the
word ideal? Recall from Unit 31 that we
discussed the kinetic molecular theory.
Within that theory, we make assumptions about gas molecules that are not
necessarily always valid to make. We
call a gas that follows these assumptions an ideal gas; when gas does not, we call it a real gas.
|
Ideal
Gas |
Real
Gas |
|
1. The molecules have no volume. 2. Collisions between molecules are elastic. 3. There is no attraction between the
molecules. |
1. Molecules do have volume. 2. Collisions between molecules are not
elastic. 3. There is an attraction between molecules. |
We use the ideal gas law because it typically
works. That is, most gases exhibit ideal
behavior under normal conditions.
And now watch this video to watch a demonstration that
illustrates the difference between the real and ideal behavior of air in a
balloon: Air Balloon
ChemLab: Ideal Gas Behavior
Overview: You will observe the movement of
particles of an ideal gas at a variety of temperatures. You will compare graphical representations of
different gas samples to consider when gases behave ideal v. real.
Directions:
1. Download the Student Exploration
and Vocabulary sheets
for the Ideal Gas Behavior.
2. Familiarize yourself with the
words on the vocabulary sheet.
3. Log-in to your Explore
Learning account.
4. Click on “Temperature and
Particle Motion” and launch the gizmo. [PLEASE NOTE: The name of the Gizmo is not the name of your
exploration sheet.]
5. Answer the Prior Knowledge
Question.
6. Practice using the Gizmo,
using the Gizmo warm-up instructions.
7.
After you are comfortable using the Gizmo, begin the activity.
Use the lab sheet as a guide to complete the 2 activities:
a. Activity A: Molecular Motions
b. Activity B: Average Particle
Velocity